Chronic obstructive pulmonary disease (COPD) is the 3rd leading cause of death in the U.S. population. Currently there are no available therapies that reverse or prevent lung damage in this heterogeneous disease process. A major area of need is the identification of mediators in blood or lung fluid that can guide development of novel effective treatments for COPD subtypes.

To address this issue, the Alexander-Brett lab, managed by Jen Alexander-Brett, MD, PhD, Associate Professor of Medicine, WashU Medicine Division of Pulmonary and Critical Care Medicine, collected and analyzed a series of human lung COPD biospecimens, including small vesicles released from cells into the airways, to facilitate discovery of cellular processes that can drive chronic inflammation, tissue damage and poor lung function. This work was recently published in the journal JCI Insight.
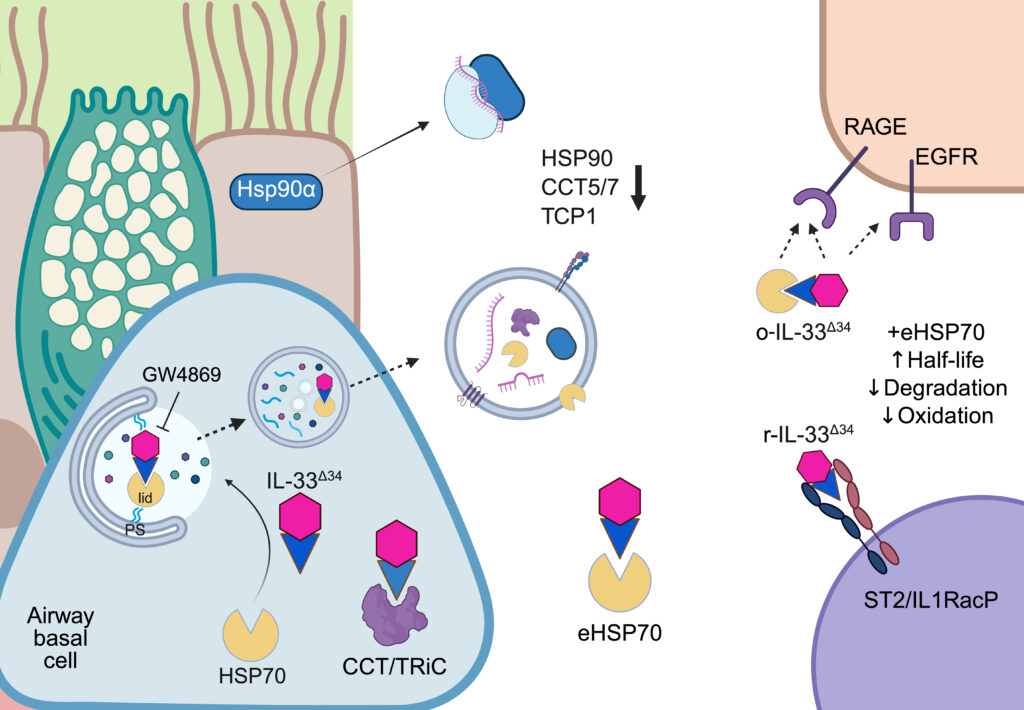
Through this analysis they learned COPD disease pathology stems in part from dysfunction in the cellular protein damage response, also called ‘proteostasis’. Specifically, they found that protein folding chaperones, including HSP70, HSP90 and the TCP1 complex, exhibit altered secretion patterns in COPD. This apparent disruption in normal cellular repair promotes inflammation driven by the key pathogenic cytokine IL-33, leading to excessive mucus production and bronchial obstruction.
“We found that the IL-33 cytokine can interact with protein refolding chaperones HSP70 and TCP1, which have the essential role of maintaining cellular health. Under disease conditions, HSP70 appears to promote secretion of the inflammatory cytokine IL-33, and once outside the cell, HSP70 can protect IL-33 from oxidation and increase the amplitude of inflammatory signaling.”
Omar Osorio, Postdoctoral Research Fellow

They found that protein folding chaperones were increasingly externalized from cells in COPD, in both soluble form and bound to secreted vesicles. They believe this phenomenon represents exhaustion of the protein folding chaperone system, which tracks with COPD disease progression, and reflects a cell’s inability to resolve inflammation. It remains to be seen whether modulating chaperone activity can be a new therapeutic avenue through revamping cellular mechanisms already tailored to maintain the cell at homeostasis. Investigating the potential for networks could therefore lead to new therapeutics for COPD and is the focus of ongoing research in the Alexander-Brett lab.
The Alexander-Brett lab would like to thank funding sources including: NIH/NHLBI (K08HL121168, R01HL152245, R01HL170198, T32HL007317), American Thoracic Society, Burroughs Wellcome Fund, Doris Duke Foundation (2015215) and the Hardy Charitable Trust.
Publication: Osorio OA, Kluender CE, Raphael, HR, Hassan, GF, Cohen LS, Steinberg DE, Kiriakos, E, Payne MD, Luo, EM, Hicks, J, Byers DE, Alexander-Brett J, “HSP70 is a chaperone for IL-33 activity in chronic airway disease” JCI Insight, Jun 10(15): e193640.